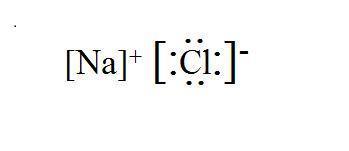NaCl Lewis Structure
In the case of NaCl:
- Sodium (Na) donates one electron to achieve a stable electron configuration like neon (Na⁺).
- Chlorine (Cl) accepts one electron to achieve a stable electron configuration like argon (Cl⁻).
Therefore, the Lewis structure of NaCl would not include shared electron pairs between sodium and chlorine atoms. Instead, it's represented as Na⁺Cl⁻ to denote the transfer of one electron from sodium to chlorine, resulting in oppositely charged ions that attract each other due to electrostatic forces to form the ionic compound sodium chloride.
2
2 reads
CURATED FROM
IDEAS CURATED BY
Given that sodium chloride (NaCl) is an ionic compound, it does not have a typical Lewis structure like covalent compounds. In ionic compounds like NaCl, the bonding occurs through the transfer of electrons from one atom to another, rather than through the sharing of electrons as in covalent bonds.
“
Read & Learn
20x Faster
without
deepstash
with
deepstash
with
deepstash
Personalized microlearning
—
100+ Learning Journeys
—
Access to 200,000+ ideas
—
Access to the mobile app
—
Unlimited idea saving
—
—
Unlimited history
—
—
Unlimited listening to ideas
—
—
Downloading & offline access
—
—
Supercharge your mind with one idea per day
Enter your email and spend 1 minute every day to learn something new.
I agree to receive email updates