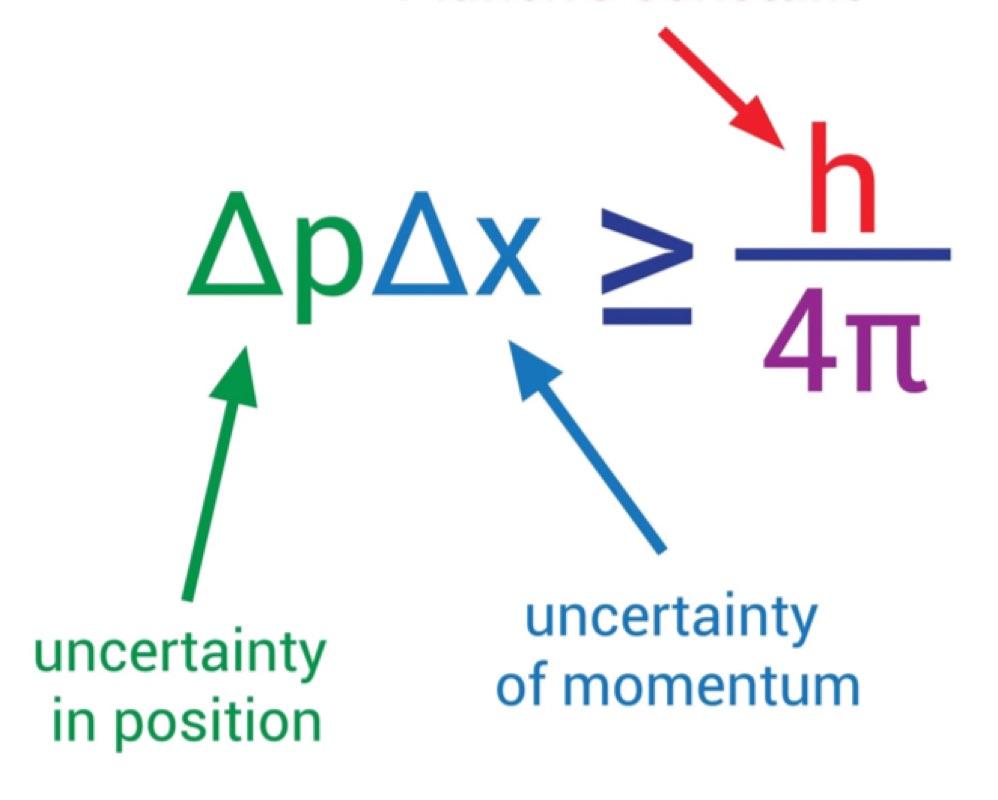
Heinsenberg Principle Of Uncertainty
The Heisenberg principle of uncertainty states that you can’t simultaneously know with high precision both the position and momentum of a particle.
If you know the position, you won’t know the exact momentum and vice-versa.
This is due to the act of measurements induced on the phenomenon being observed.
For example, if we use a specific laser to know the exact position of an atom, the laser would also influence the momentum and we won’t know it.
Translation in everyday life:
Focusing solely on one thing, will distort your ability to have accurate information on the other things.
71
581 reads
CURATED FROM
IDEAS CURATED BY
Quantum Physics simplified
“
Similar ideas to Heinsenberg Principle Of Uncertainty
Heisenberg’s Uncertainty Principle
- “It is impossible to design an apparatus to determine which hole the electron passes through, that will not at the same time disturb the electrons enough to destroy the interference pattern.”
- “One cannot know both where something is and how fast it is moving.”
- The uncertai...
Read & Learn
20x Faster
without
deepstash
with
deepstash
with
deepstash
Personalized microlearning
—
100+ Learning Journeys
—
Access to 200,000+ ideas
—
Access to the mobile app
—
Unlimited idea saving
—
—
Unlimited history
—
—
Unlimited listening to ideas
—
—
Downloading & offline access
—
—
Supercharge your mind with one idea per day
Enter your email and spend 1 minute every day to learn something new.
I agree to receive email updates