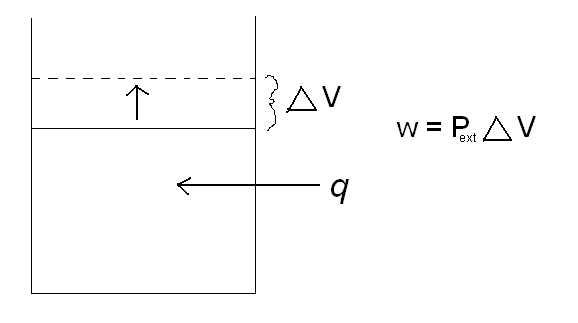
Equation explained
where Pext is the external pressure, and 🔺V is the change in volume. A classic example of this is a piston. As heat is added to the cylinder, the pressure inside the cylinder increases. The piston then rises to relieve the pressure difference between the pressure inside the cylinder and the external pressure. By increasing the volume in the cylinder, the piston has just done work. Reference the picture below.
The sum of heat and work is the change in internal energy, Δ U .
In an isolated system, q = − w . Therefore, Δ U = 0 .
12
35 reads
CURATED FROM
IDEAS CURATED BY
Highschool Student who has passionate interest in different sciences and World history.
The idea is part of this collection:
Learn more about scienceandnature with this collection
The differences between Web 2.0 and Web 3.0
The future of the internet
Understanding the potential of Web 3.0
Related collections
Similar ideas to Equation explained
Thermochemistry basics
The law of Conservation of Energy refers to an isolated system in which there is no net change in energy and where energy is neither created nor destroyed. Although there is no change in energy, energy can change forms, for example from potential to kinetic energy. In ot...
Read & Learn
20x Faster
without
deepstash
with
deepstash
with
deepstash
Personalized microlearning
—
100+ Learning Journeys
—
Access to 200,000+ ideas
—
Access to the mobile app
—
Unlimited idea saving
—
—
Unlimited history
—
—
Unlimited listening to ideas
—
—
Downloading & offline access
—
—
Supercharge your mind with one idea per day
Enter your email and spend 1 minute every day to learn something new.
I agree to receive email updates