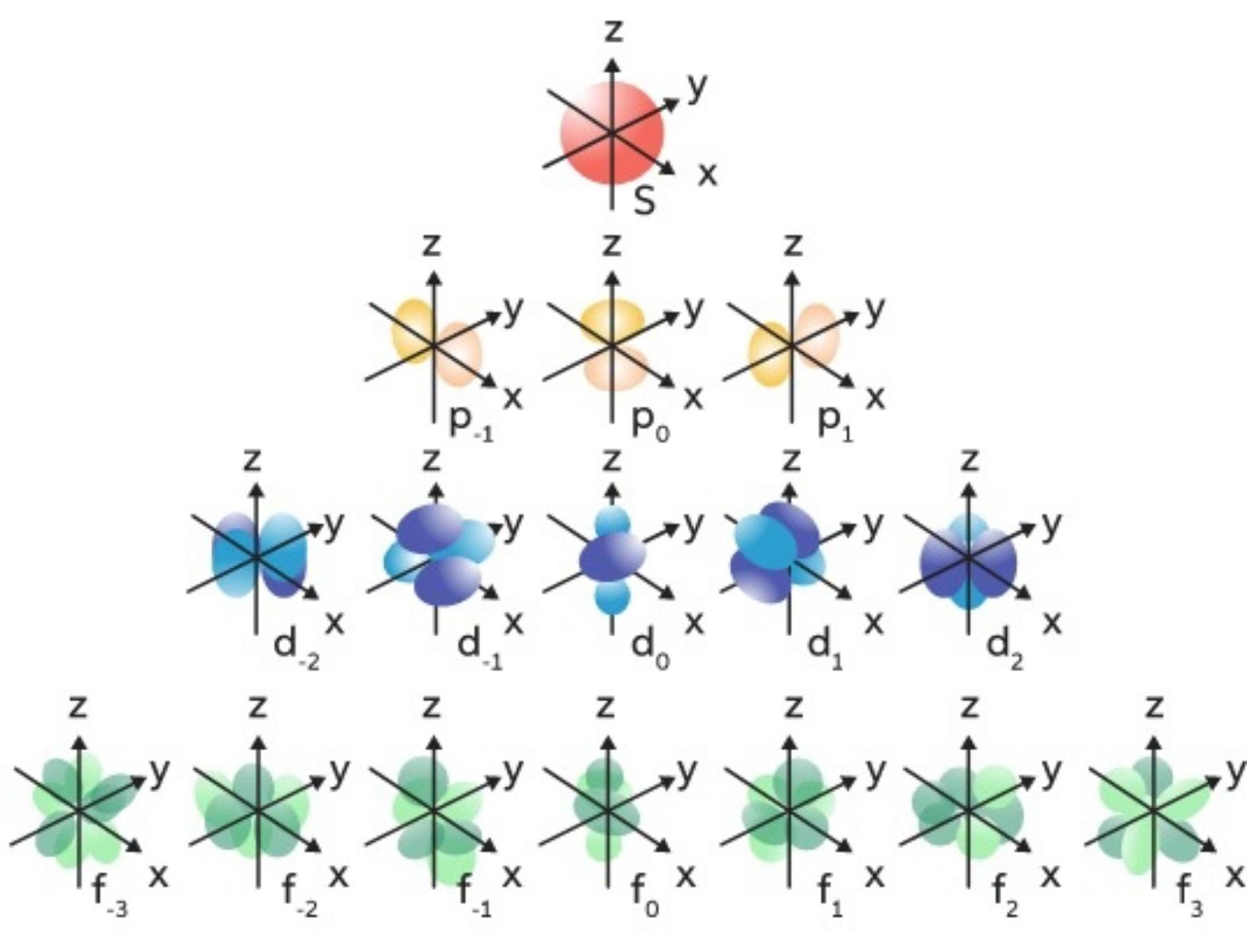
Magnetic quantum Numbers (mℓ)
Specifie the orientation in space of an orbital of a given energy (n) and shape (l). This number divides the subshell into individual orbitals which hold the electrons; there are 2l+1 orbitals in each subshell.
Every orbital capacity is 2 electrons
Magnetic values are {-3,-2,-1,0,1,2,3}
- S ==> 1 orb ==> 0
- P ==> 3 orbs ==> -1,0,1
- D ==> 5 orbs ==> -2,-1,0,1,2
- F ==> 7 orbs ==> -3,-2,-1,0,1,2,3
photo owner: unknown
25
66 reads
CURATED FROM
IDEAS CURATED BY
Highschool Student who has passionate interest in different sciences and World history.
The idea is part of this collection:
Learn more about scienceandnature with this collection
Basic survival skills
How to prioritize needs in survival situations
How to adapt to extreme situations
Related collections
Similar ideas to Magnetic quantum Numbers (mℓ)
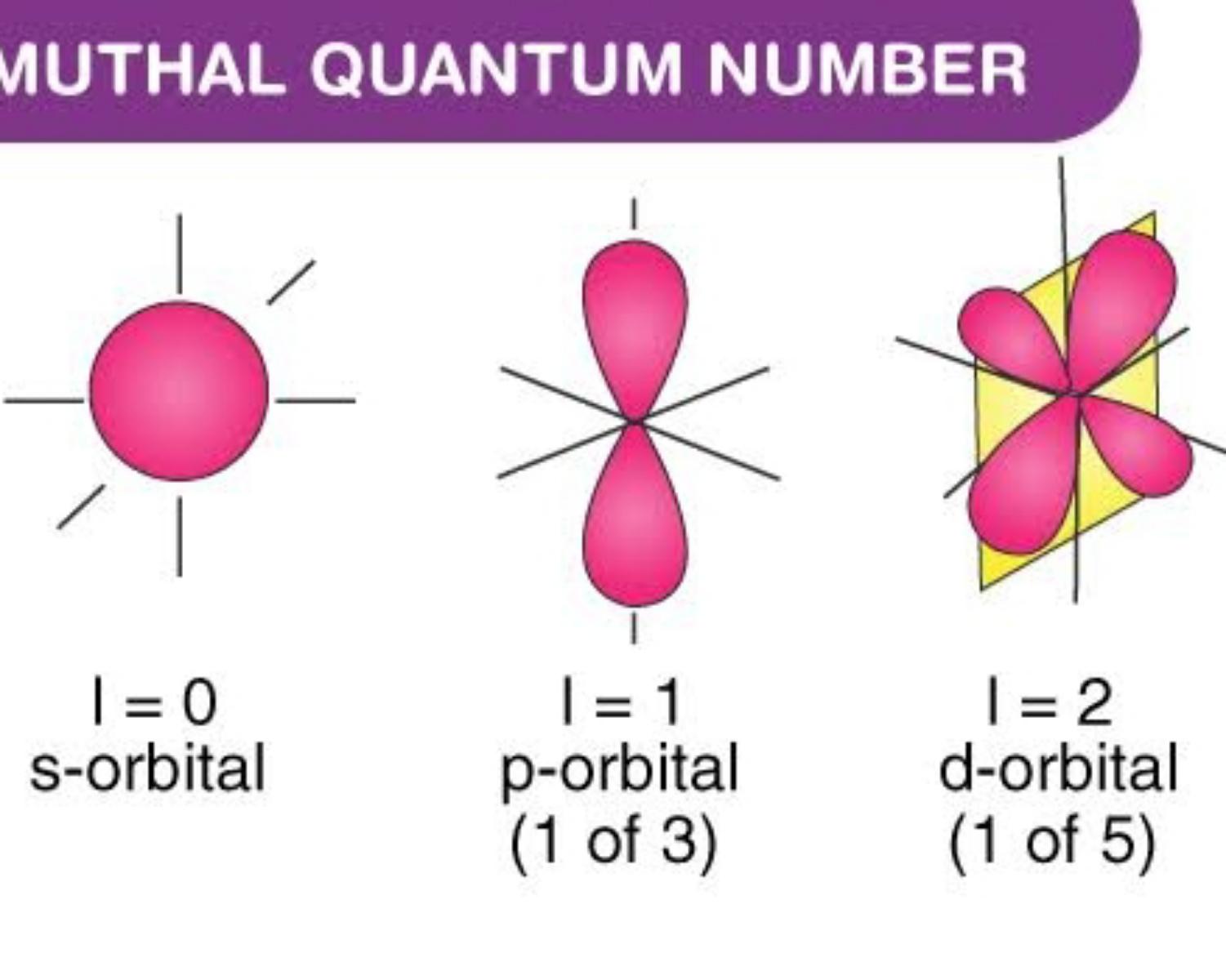
Azimuthal Quantum Numbers (ℓ)
The azimuthal quantum number is a quantum number for an atomic orbital that determines its orbital angular momentum and describes the shape of the orbital.
Every azimuthal number has his own capacity of electrons
- S(v=0) ==> 1 orb ==> 2 electrons
- ...
Read & Learn
20x Faster
without
deepstash
with
deepstash
with
deepstash
Personalized microlearning
—
100+ Learning Journeys
—
Access to 200,000+ ideas
—
Access to the mobile app
—
Unlimited idea saving
—
—
Unlimited history
—
—
Unlimited listening to ideas
—
—
Downloading & offline access
—
—
Supercharge your mind with one idea per day
Enter your email and spend 1 minute every day to learn something new.
I agree to receive email updates