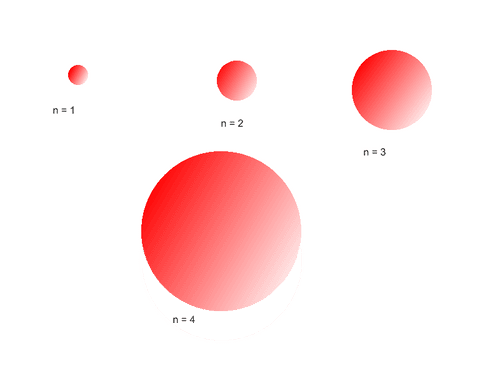Explore the World's Best Ideas
Join today and uncover 100+ curated journeys from 50+ topics. Unlock access to our mobile app with extensive features.
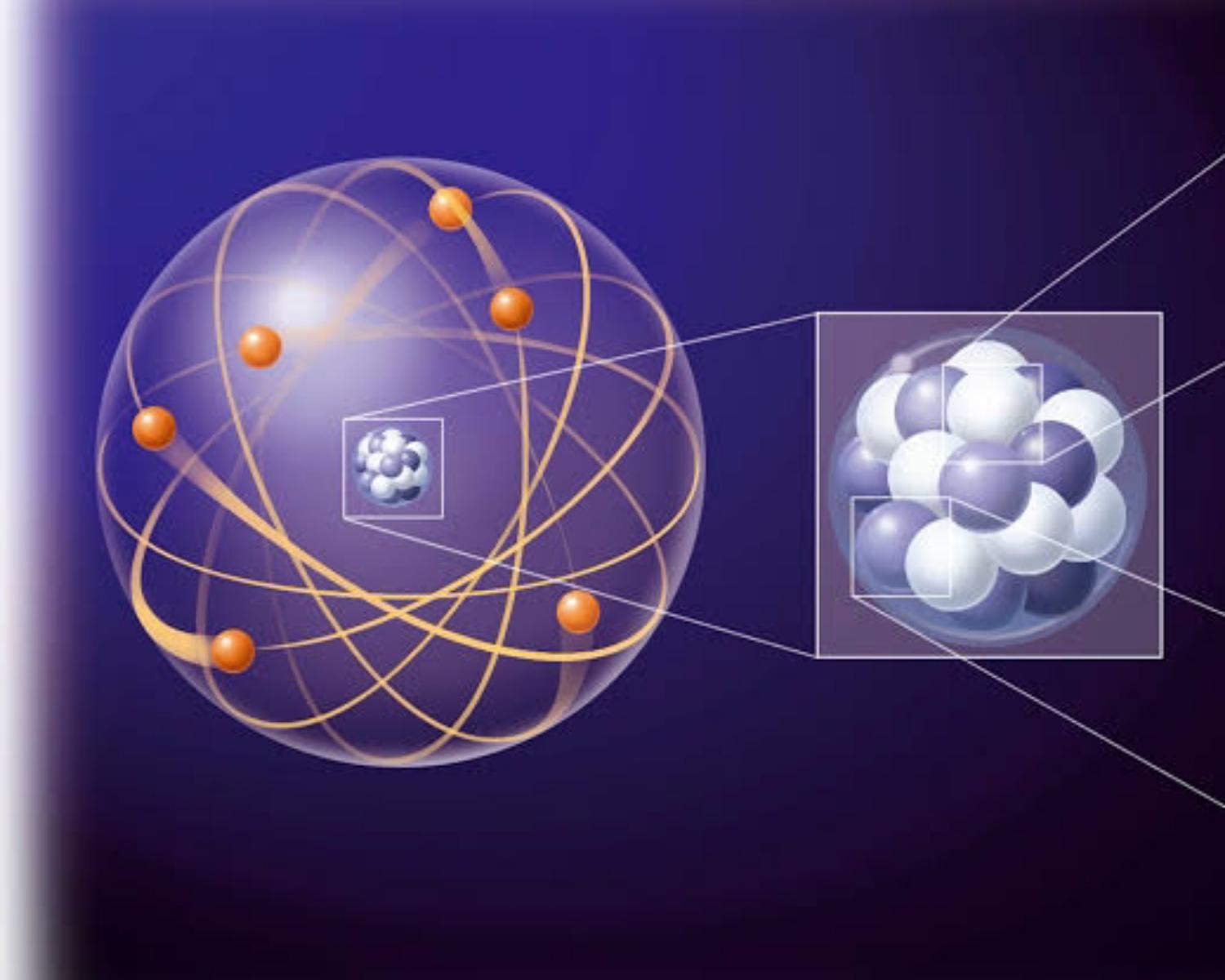
What Is A Quantum Number
A quantum number is a value that is used when describing the energy levels available to atoms and molecules.
It defines the exact position of electron in atom.
photo owner: Universiteit Leiden
31
247 reads
Quantum Numbers
There is four quantum numbers that are used to describe completely the movement and trajectories of each electron within an atom.
- Principal
- Azimuthal
- Magnetic
- Spin
photo owner: ThoughCo
26
123 reads
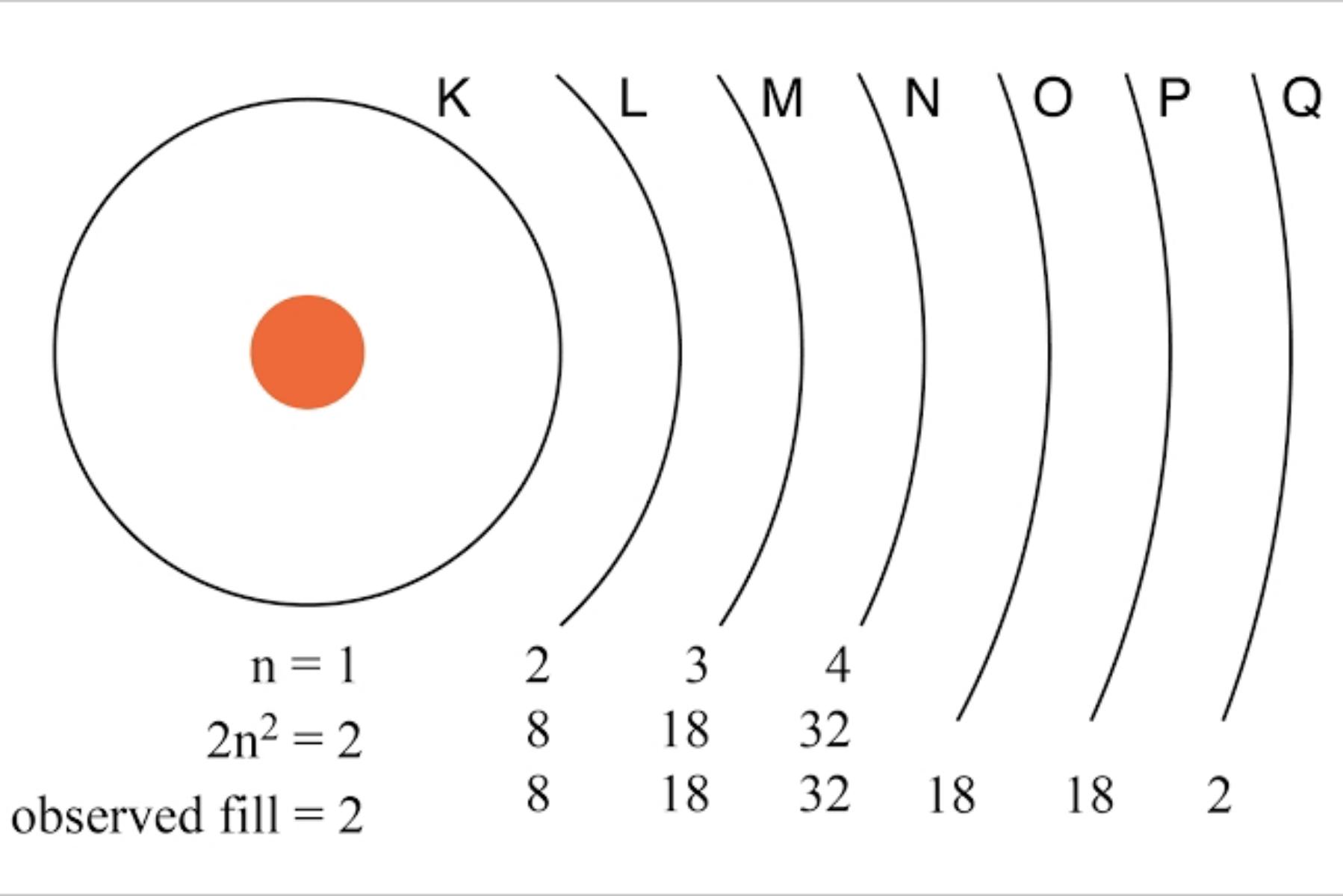
Principal Quantum Numbers (n)
describe the energy of an electron and the most probable distance of the electron from the nucleus.
To know the maximum amount of electron that an energy level can handle, Use this formula 2n² (does not include O , P , Q).
photo owner: All About Circuits
25
107 reads
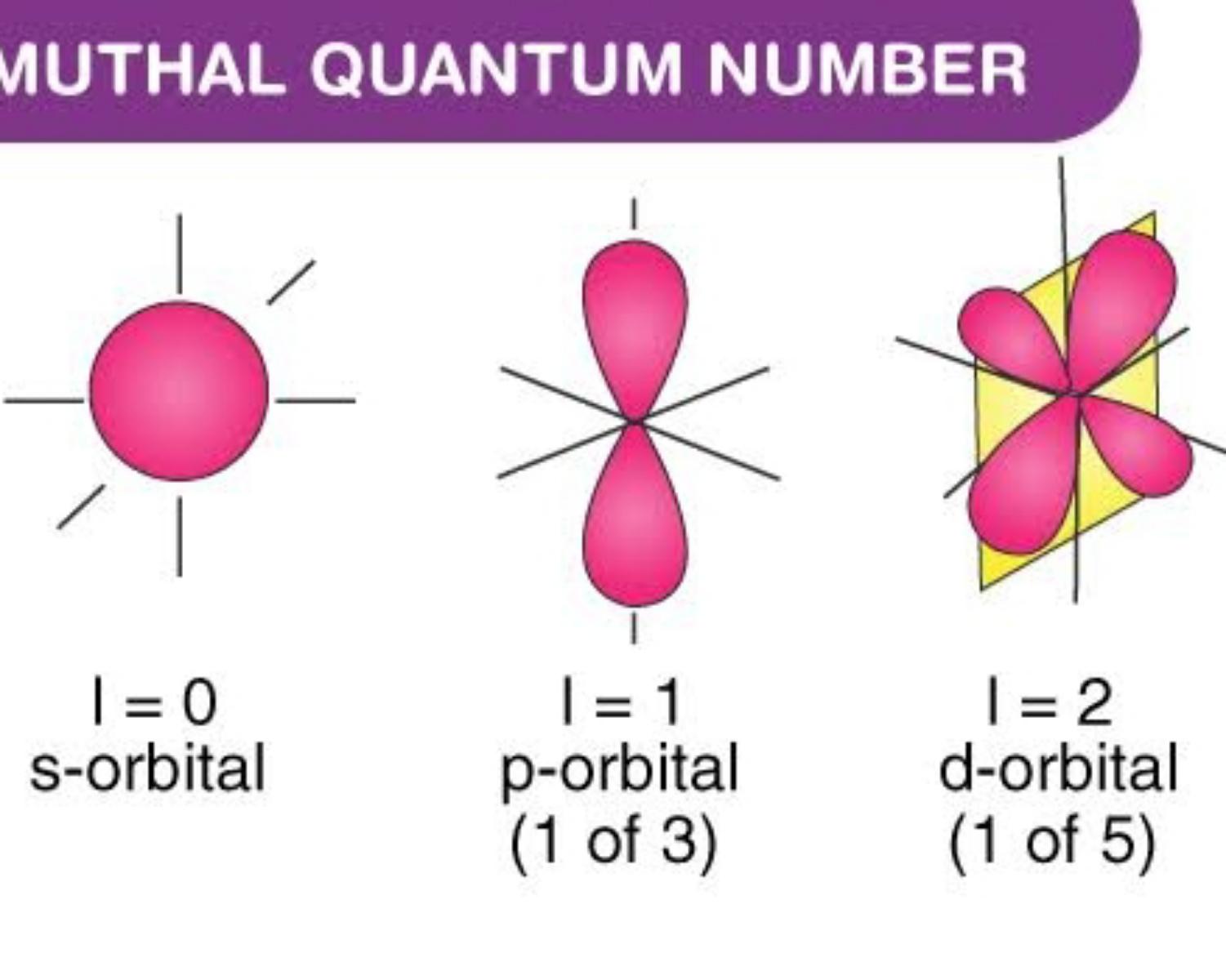
Azimuthal Quantum Numbers (ℓ)
The azimuthal quantum number is a quantum number for an atomic orbital that determines its orbital angular momentum and describes the shape of the orbital.
Every azimuthal number has his own capacity of electrons
- S(v=0) ==> 1 orb ==> 2 electrons
- P(v=1) ==> 3 orbs ==> 6 electrons
- D(v=2) ==> 5 orbs ==> 10 electrons
- F(v=3) ==> 10 orbs ==>12 electrons
Ps:open the photo to see the whole image. ((v = value))
photo owner: Byju's
25
65 reads
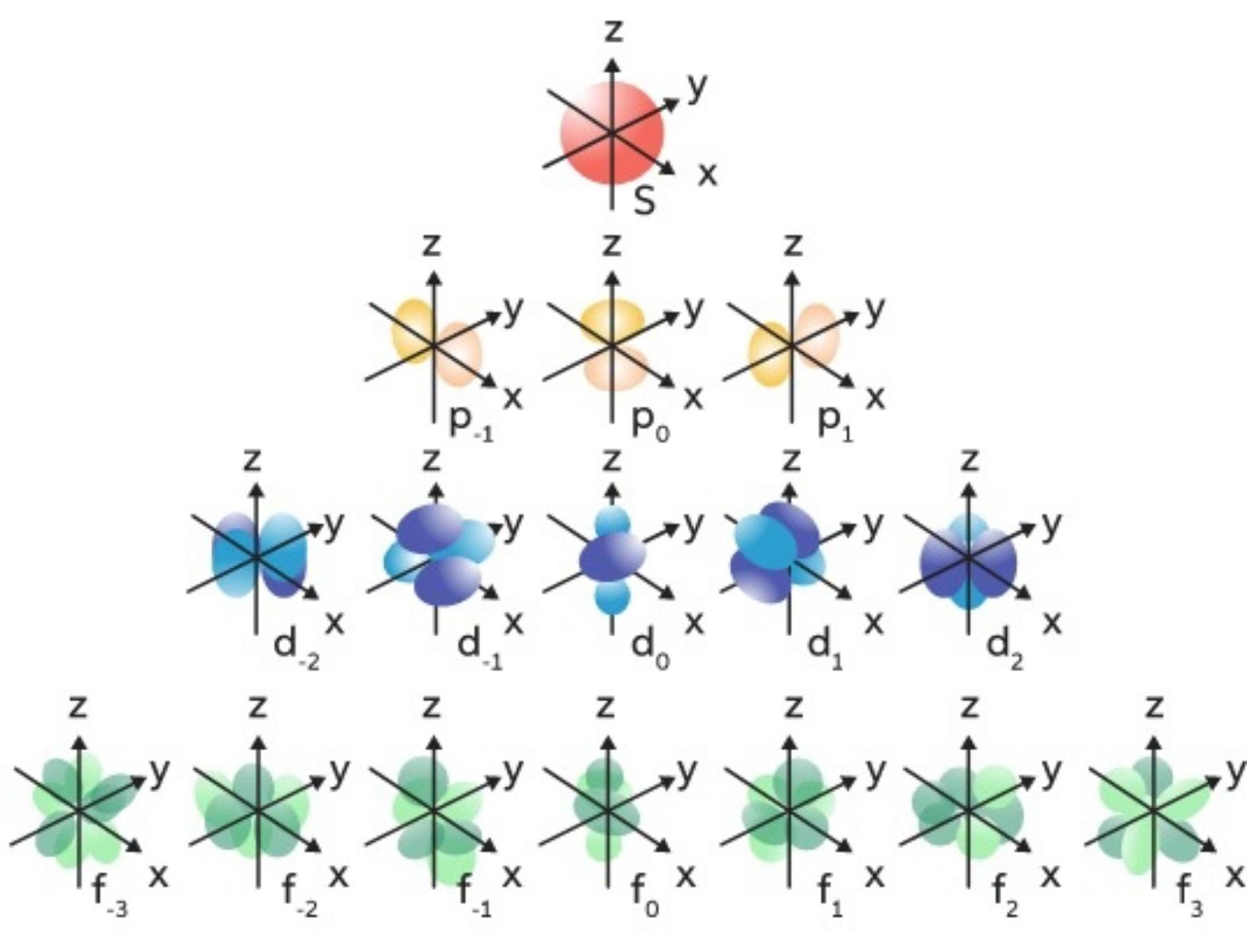
Magnetic quantum Numbers (mℓ)
Specifie the orientation in space of an orbital of a given energy (n) and shape (l). This number divides the subshell into individual orbitals which hold the electrons; there are 2l+1 orbitals in each subshell.
Every orbital capacity is 2 electrons
Magnetic values are {-3,-2,-1,0,1,2,3}
- S ==> 1 orb ==> 0
- P ==> 3 orbs ==> -1,0,1
- D ==> 5 orbs ==> -2,-1,0,1,2
- F ==> 7 orbs ==> -3,-2,-1,0,1,2,3
photo owner: unknown
25
66 reads
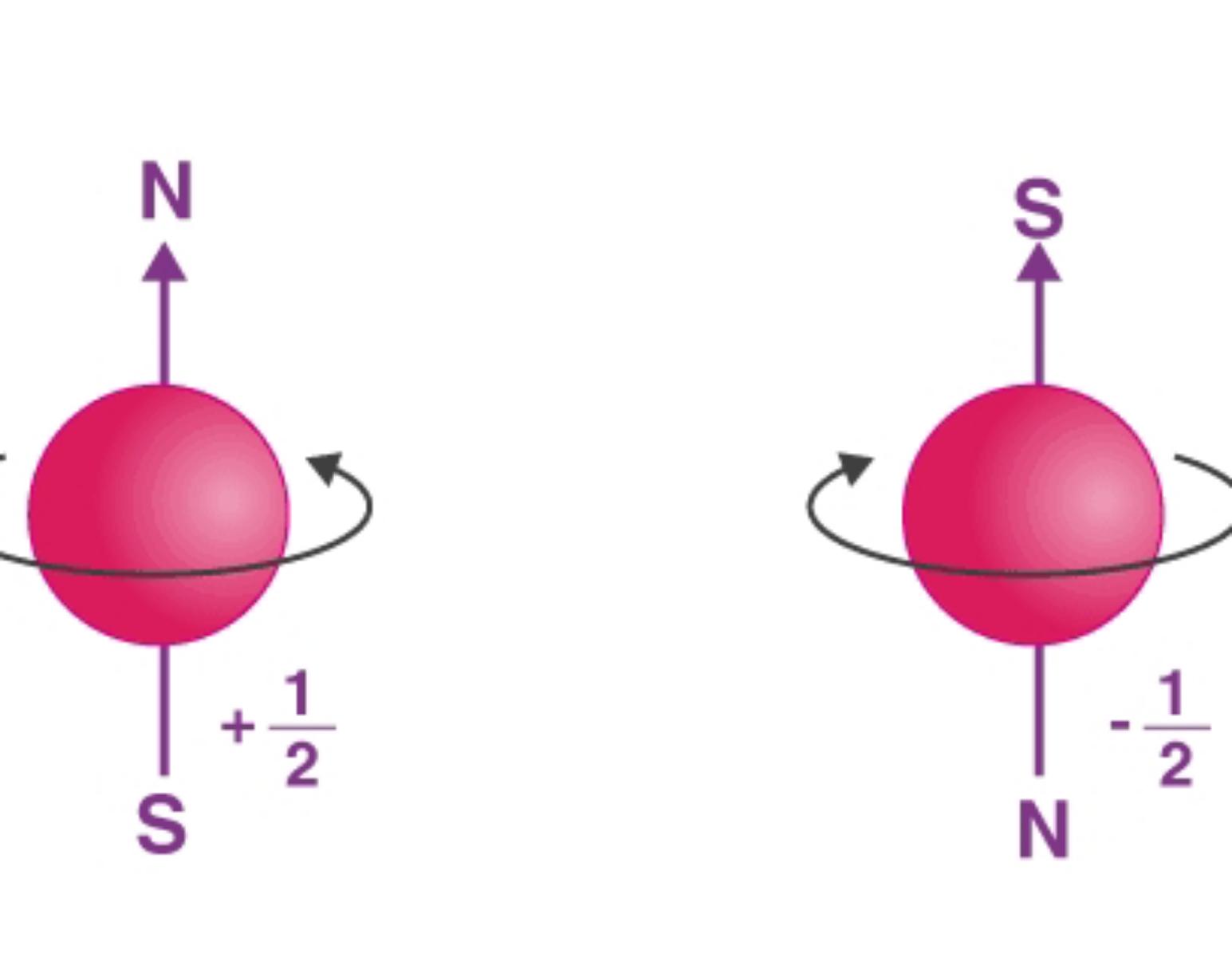
Spin Quantum Numbers (ms)
describe the angular momentum of an electron. An electron spins around an axis and has both angular momentum and orbital angular momentum. Because angular momentum is a vector, the Spin Quantum Number (s) has both a magnitude (½) and direction (+ or -)
(+½) ===> in the direction of clock
(-½) ===> against the direction of clock
The electrons take place in orbs in a specific way.
Imagine the orbs like beds and electrons like persons. Every person tend to sleep alone in bed (+½) unless there is no bed left so the other person have to sleep upside down (-½).
photo owner: Byju's
26
52 reads
Practice
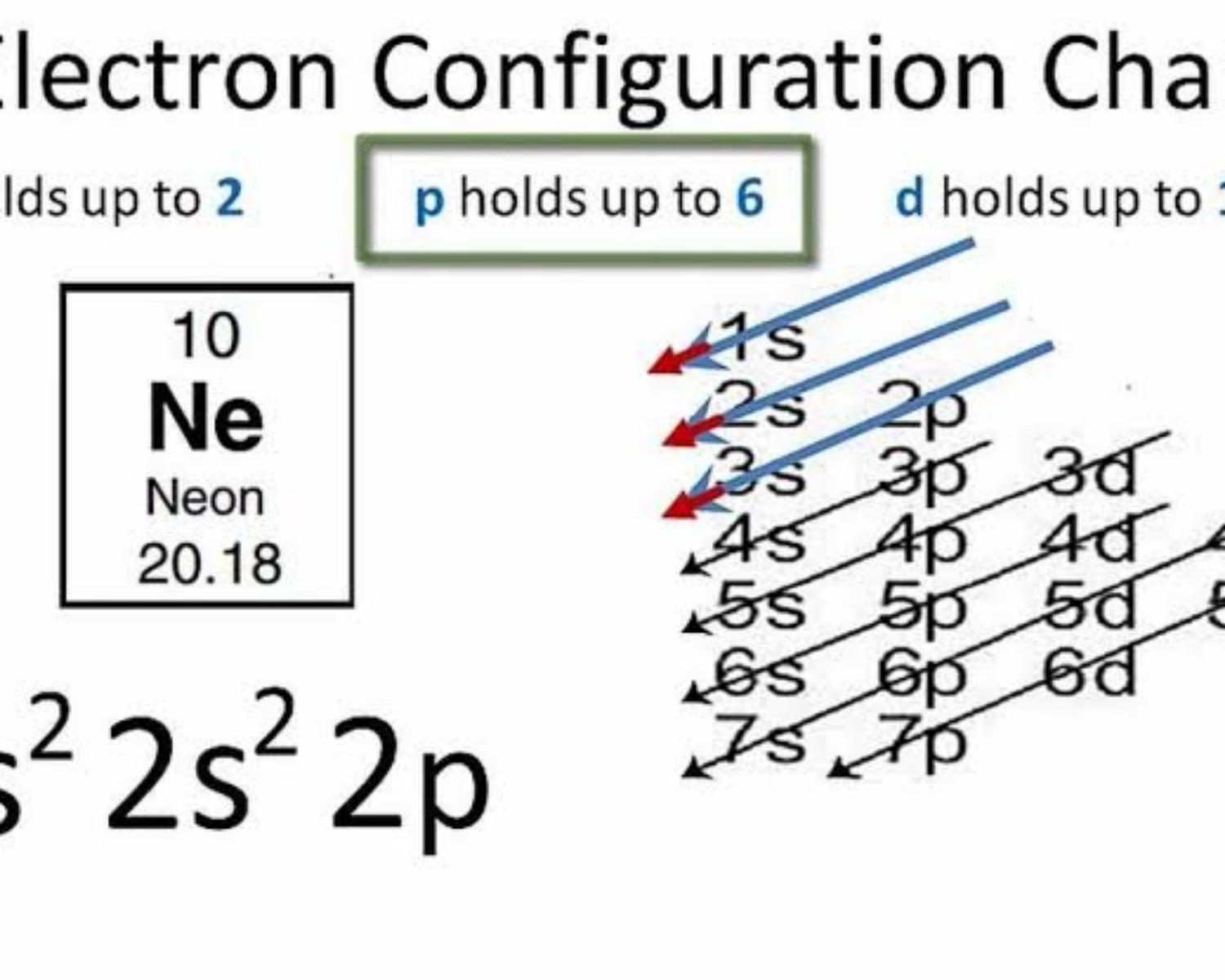
We have to define the exact place of the last electron for the element Neon¹⁰
- We have to configure the electrons 1s²,2s²,2p⁶
- Now we focus on the last number 2p⁶
• n (the number before the letter) = 2
• ℓ ( = P ) = 1
3. We define P's orbitals
2p⁶ ==> 3 orbs ==> 6 electrons
Every orb contains 2 electrons
4. We have to define spin
|(+½)¹ (-½)⁴|.|(+½)² (-½)⁵ |.|(+½)³ (-½)⁶|.
-----[-1]------. -----[0]------. -----[1]------
The order of spin is marked on top of the electron¹
• mℓ (last electron place) = 1
• ms = -½
Hope you enjoy the explanation :)
See you in the next post 👋.
25
55 reads
IDEAS CURATED BY
Highschool Student who has passionate interest in different sciences and World history.
Ziad Mohsen's ideas are part of this journey:
Learn more about scienceandnature with this collection
Basic survival skills
How to prioritize needs in survival situations
How to adapt to extreme situations
Related collections
Similar ideas
17 ideas
A BUTTERFLY IN THE QUANTUM WORLD
canblogum.medium.com
2 ideas
What is an Atom?
livescience.com
1 idea
Quantum entanglement - Wikipedia
en.m.wikipedia.org
Read & Learn
20x Faster
without
deepstash
with
deepstash
with
deepstash
Personalized microlearning
—
100+ Learning Journeys
—
Access to 200,000+ ideas
—
Access to the mobile app
—
Unlimited idea saving
—
—
Unlimited history
—
—
Unlimited listening to ideas
—
—
Downloading & offline access
—
—
Supercharge your mind with one idea per day
Enter your email and spend 1 minute every day to learn something new.
I agree to receive email updates