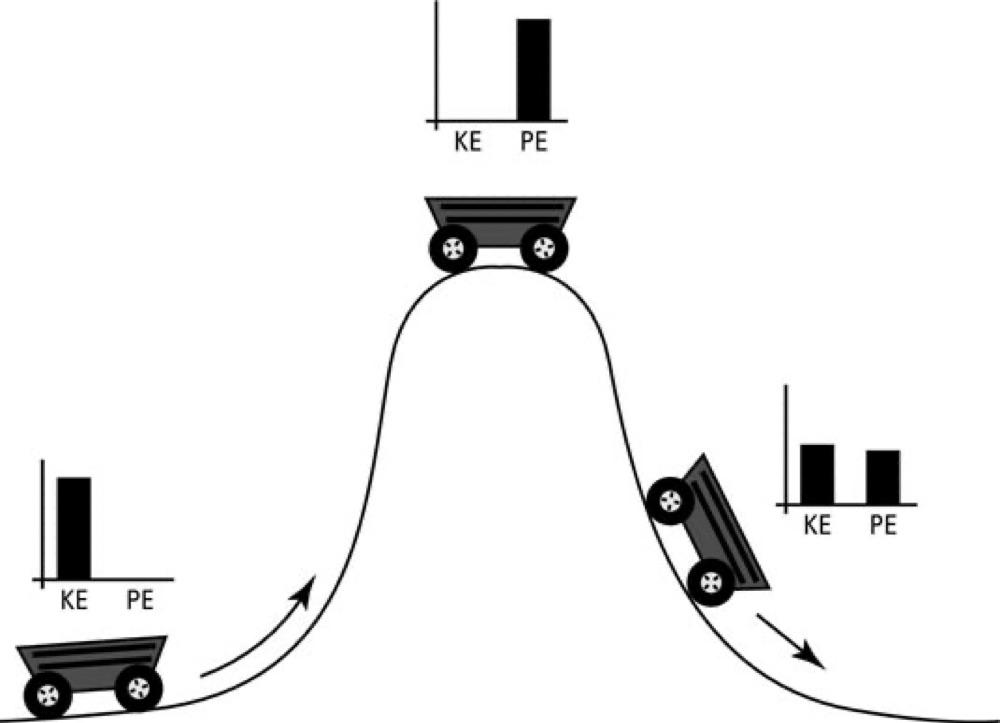
CONSERVATION OF ENERGY
- The energy has a large number of different forms, and there is a formula for each one. These are gravitational energy, kinetic energy, heat energy, elastic energy, electrical energy, chemical energy, radiant energy, nuclear energy, mass energy.
- If we total up the formulas for each of these contributions, it will not change except for energy going in and out.
349
1.22K reads
CURATED FROM
IDEAS CURATED BY
The more one seeks to rise into height and light, the more vigorously do ones roots struggle earthward, downward, into the dark, the deep — into evil.
Richard Phillips Feynman (May 11, 1918 – February 15, 1988) was an American theoretical physicist, known for his work in the path integral formulation of quantum mechanics, the theory of quantum electrodynamics, the physics of the superfluidity of supercooled liquid helium, as well as his work in particle physics for which he proposed the parton model. For his contributions to the development of quantum electrodynamics, Feynman received the Nobel Prize in Physics in 1965 jointly with Julian Schwinger and Shin'ichirō Tomonaga.
“
The idea is part of this collection:
Learn more about books with this collection
How to create customer-centric strategies
The importance of empathy in customer success
The impact of customer success on business growth
Related collections
Similar ideas to CONSERVATION OF ENERGY
The different forms of energy
- Heat - results from movement of atoms; considered as energy in due to relations to temperature
- Kinetic - energy of motion
- Potential - energy due to an object's position
- Mechanical - the sum of kin...
The Law of Conservation Energy
According to the law of conservation of energy, the total energy of a system remains constant through the energy that may change into another form.
Moreover, energy is something that cannot be created not destroyed, it simply is. Energy can change forms and is als...
Thermochemistry basics
The law of Conservation of Energy refers to an isolated system in which there is no net change in energy and where energy is neither created nor destroyed. Although there is no change in energy, energy can change forms, for example from potential to kinetic energy. In ot...
Read & Learn
20x Faster
without
deepstash
with
deepstash
with
deepstash
Personalized microlearning
—
100+ Learning Journeys
—
Access to 200,000+ ideas
—
Access to the mobile app
—
Unlimited idea saving
—
—
Unlimited history
—
—
Unlimited listening to ideas
—
—
Downloading & offline access
—
—
Supercharge your mind with one idea per day
Enter your email and spend 1 minute every day to learn something new.
I agree to receive email updates