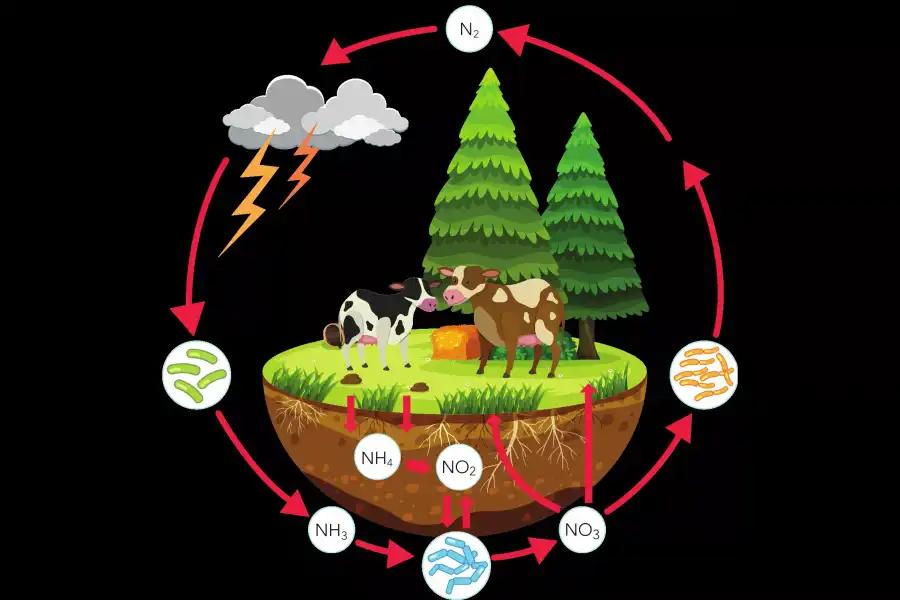
The Nitrogen Cycle
Nitrogen is an essential component of biological molecules like amino and nucleic acids, but most living organisms cannot use the element in its pure form and need a derivative component, NH3(ammonia) to be able to synthesize organic components.
84
210 reads
CURATED FROM
IDEAS CURATED BY
The idea is part of this collection:
Learn more about personaldevelopment with this collection
Cultivating a growth mindset and embracing challenges
Developing adaptive thinking and problem-solving skills
Effective learning frameworks and approaches
Related collections
Similar ideas to The Nitrogen Cycle
Steps Of the Nitrogen Cycle
- Nitrogen (N2) is converted in ammonia (NH3) by bacteria in water and soil, and subsequently converted into nitrite and its derivatives.
- Plants get nitrogen from the soil through the roots, producing organic compounds.
- Animals absorb nitrogen by eating the plants.
- ...
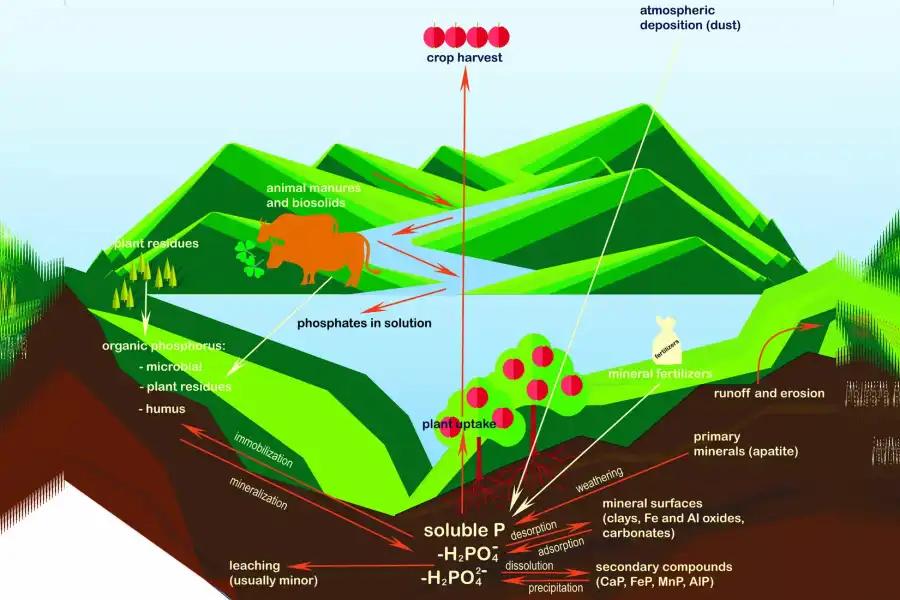
The Phosphorus Cycle
A component of certain biological molecules like phospholipids, DNA and adenosine triphosphate (ATP), phosphorus is circulated in the environment through the soil, water rocks and living organisms.
It is found organically as phosphate ion and is added to the soil and water through natural ...
7 Things You Need To Understand About Anxiety And Depression
No, we are not over yet. After discussing the topic with my friend’s Dad (he is a doctor), I’ve made a list of things you need to understand and avoid anxiety attacks (hard to breathe moments).
- Think of your anxiety as a biological problem, rather than thinking there is something wron...
Read & Learn
20x Faster
without
deepstash
with
deepstash
with
deepstash
Personalized microlearning
—
100+ Learning Journeys
—
Access to 200,000+ ideas
—
Access to the mobile app
—
Unlimited idea saving
—
—
Unlimited history
—
—
Unlimited listening to ideas
—
—
Downloading & offline access
—
—
Supercharge your mind with one idea per day
Enter your email and spend 1 minute every day to learn something new.
I agree to receive email updates