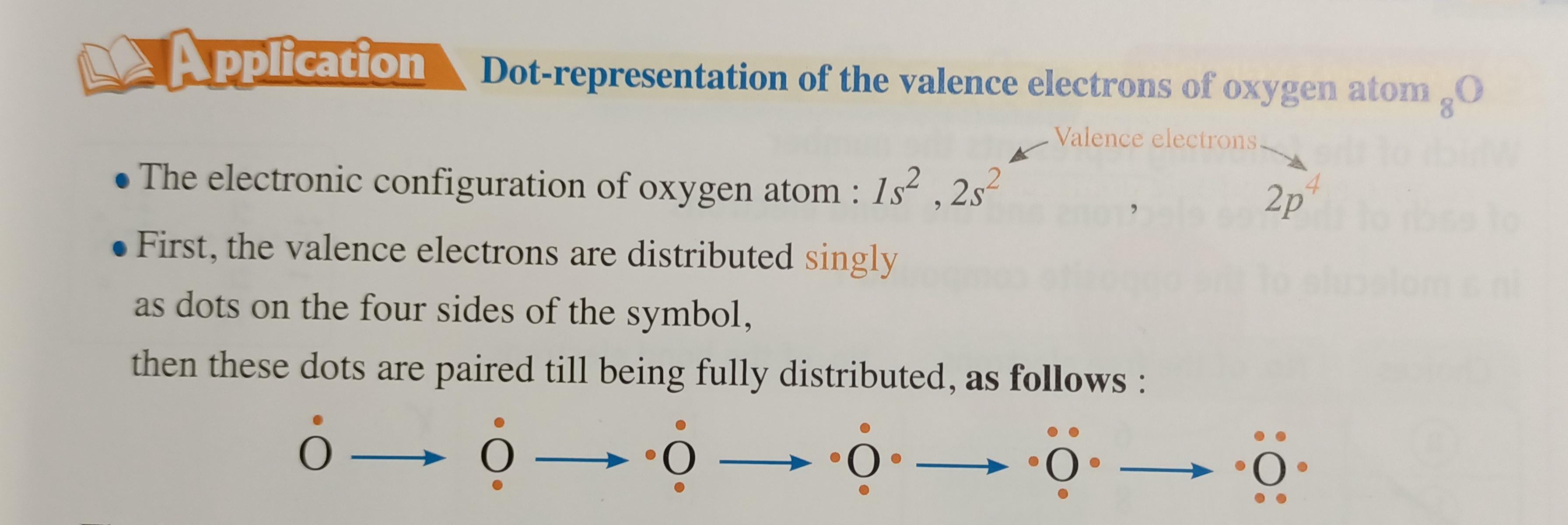Explore the World's Best Ideas
Join today and uncover 100+ curated journeys from 50+ topics. Unlock access to our mobile app with extensive features.
Must Known To Understand
There is 2 types of elements when it comes to chemical reaction in our nature : Noble Gases , Reactive elements ( all of the other elements).
- Noble Gases :
The most stable elements which don't undergo any chemical reaction (at normal condition) with other elements or with each other.
Because their outermost energy level is completely filled with electrons.
Their molecules are mono-atomic such as (He,Ne,Ar,Kr,Xe,Rn)
- Reactive Elements :
All other elements are reactive to some extent, they undergo chemical reactions to complete their outermost shell by accepting , losing , sharing electrons.
8
116 reads
Lewis Electron-dot Symbols
The Valence electrons play an important role in the formation of the bonds. So the scientist G.N.Lewis introduced a simple method to represent the valence electrons by using dots
You can open the picture to understand more about the steps.
Resource: El moasser book
8
128 reads
Types Of Bonds
Chemical Bonds Physical Bonds
1. Ionic Bonds 1. Hydrogen Bond
2. Coordinate bond 2. Metallic bond
3. Covalent Bond:-
- pure covalent bond
- Non-polar covalent bond
- Polar covalent bond
As you can see there are 7 bonds which I will explain in details in the next post .
Hope you enjoyed my explanation and found it easy to understand.
P.S "don't worry I am working on it and it will be posted later this month ;) "
9
68 reads
IDEAS CURATED BY
Highschool Student who has passionate interest in different sciences and World history.
CURATOR'S NOTE
Some people find Chemistry so complicated and confusing . So I , as a passionate high school student about Chemistry , try at my best to clarify some basics with simple explanation.
“
Read & Learn
20x Faster
without
deepstash
with
deepstash
with
deepstash
Personalized microlearning
—
100+ Learning Journeys
—
Access to 200,000+ ideas
—
Access to the mobile app
—
Unlimited idea saving
—
—
Unlimited history
—
—
Unlimited listening to ideas
—
—
Downloading & offline access
—
—
Supercharge your mind with one idea per day
Enter your email and spend 1 minute every day to learn something new.
I agree to receive email updates