What Is A Quantum Number
A quantum number is a value that is used when describing the energy levels available to atoms and molecules.
It defines the exact position of electron in atom.
photo owner: Universiteit Leiden
31
247 reads
CURATED FROM
IDEAS CURATED BY
Highschool Student who has passionate interest in different sciences and World history.
The idea is part of this collection:
Learn more about scienceandnature with this collection
Basic survival skills
How to prioritize needs in survival situations
How to adapt to extreme situations
Related collections
Similar ideas to What Is A Quantum Number
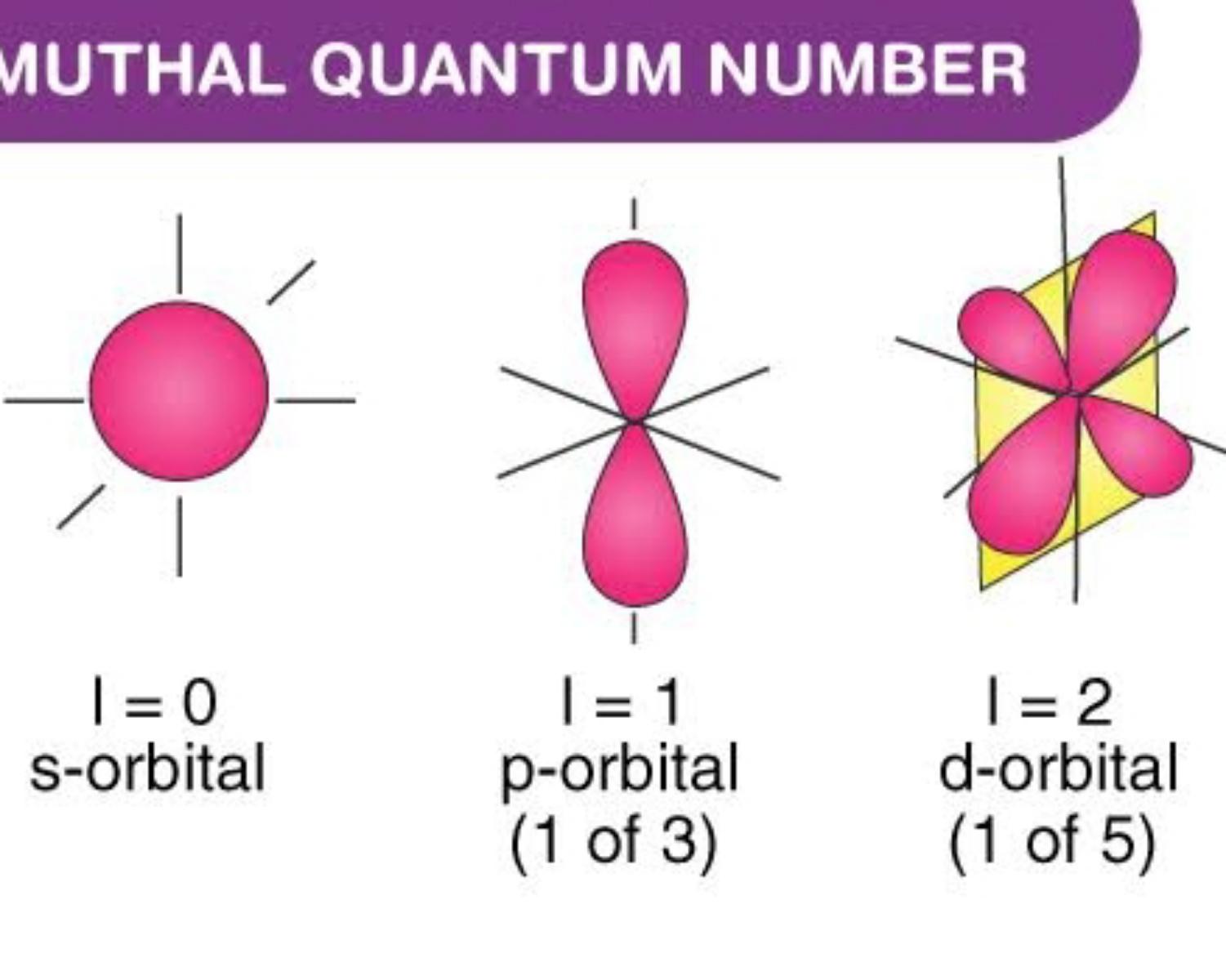
Azimuthal Quantum Numbers (ℓ)
The azimuthal quantum number is a quantum number for an atomic orbital that determines its orbital angular momentum and describes the shape of the orbital.
Every azimuthal number has his own capacity of electrons
- S(v=0) ==> 1 orb ==> 2 electrons
- ...
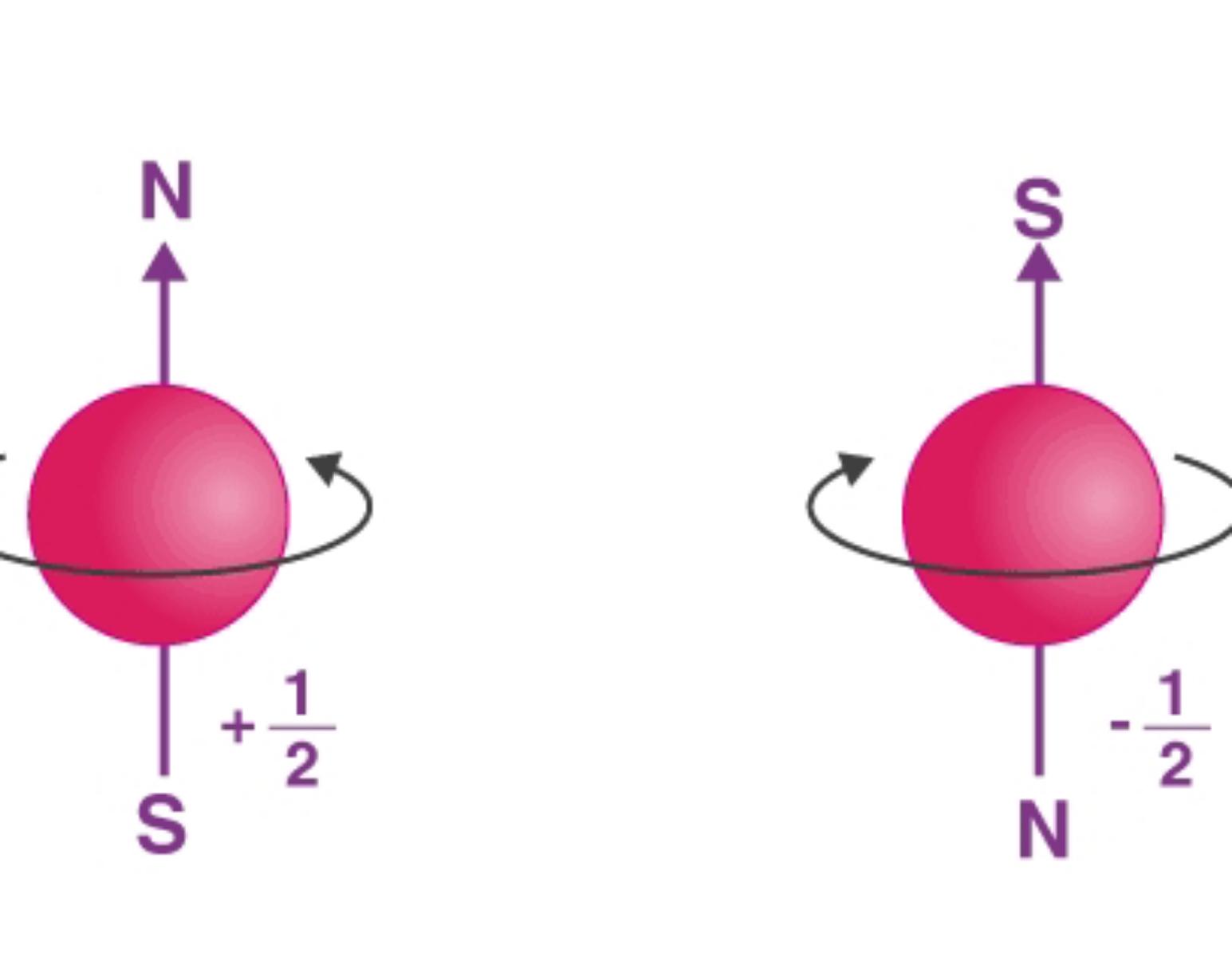
Spin Quantum Numbers (ms)
describe the angular momentum of an electron. An electron spins around an axis and has both angular momentum and orbital angular momentum. Because angular momentum is a vector, the Spin Quantum Number (s) has both a magnitude (½) and direction (+ or -)
(+½) ===> in the dire...
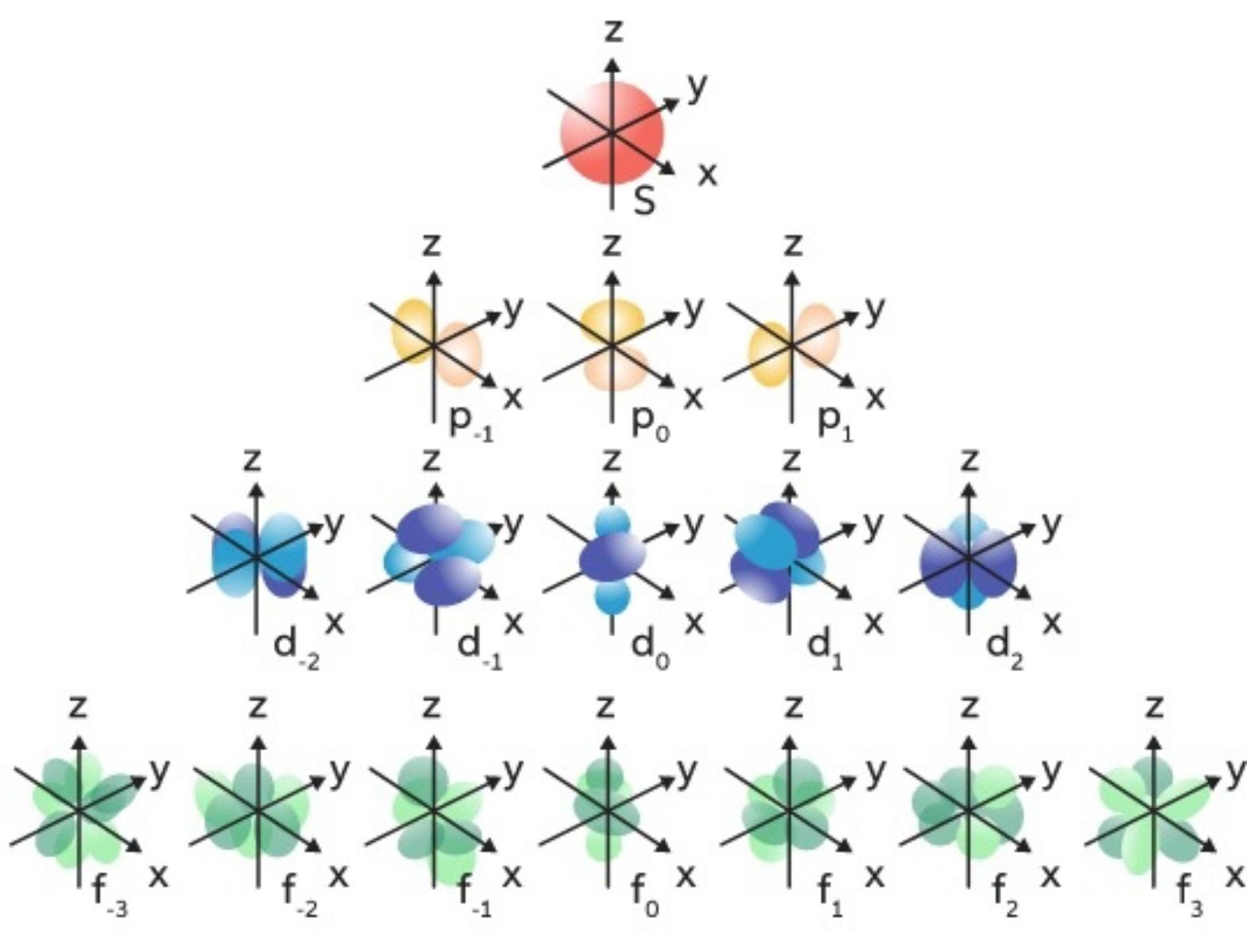
Magnetic quantum Numbers (mℓ)
Specifie the orientation in space of an orbital of a given energy (n) and shape (l). This number divides the subshell into individual orbitals which hold the electrons; there are 2l+1 orbitals in each subshell.
Every orbital capacity is 2 electrons
Magnetic ...
Read & Learn
20x Faster
without
deepstash
with
deepstash
with
deepstash
Personalized microlearning
—
100+ Learning Journeys
—
Access to 200,000+ ideas
—
Access to the mobile app
—
Unlimited idea saving
—
—
Unlimited history
—
—
Unlimited listening to ideas
—
—
Downloading & offline access
—
—
Supercharge your mind with one idea per day
Enter your email and spend 1 minute every day to learn something new.
I agree to receive email updates