Exploring the Depths of Respiration
Ideas, facts & insights covering these topics:
16 ideas
·1.59K reads
7
3
Explore the World's Best Ideas
Join today and uncover 100+ curated journeys from 50+ topics. Unlock access to our mobile app with extensive features.
Respiration
Respiration is the exchange of gases, primarily oxygen and carbon dioxide, and the utilization of the inhaled oxygen to combust digested food, releasing energy.
However, people often associate respiration solely with gaseous exchange.
But it also involves cellular respiration.
13
253 reads
The sun is the main source of energy on this planet. The light energy from the sun is transduced by plants into chemical energy. They trap this light energy in the chemical bonds of organic molecules, like in glucose.
And when we eat them, we break these bonds of organic molecules and derive the energy. Our cells use a similar mechanism for storing this energy. We store it in the chemical bonds of ATP [Adenosine triphosphate] and other compounds.
ULTIMATE ENERGY SOURCE - SUN
12
189 reads
Food - We Eat Gives Us Energy; But How?
We might wonder how the food we eat provides us with energy. In what way is energy derived from the same food material?
We consume food, which is broken down into smaller components and eventually into their respective smaller building blocks.
These building blocks are absorbed into the body's fluids and eventually reach the cells.
13
163 reads
What Happens in Cells?
Once nutrients, including carbohydrates, proteins, fats, and more, reach the cell, they are utilized by cells to produce energy.
For example, when sucrose (cane sugar) reaches the cell, it is initially hydrolyzed into fructose and glucose. These two compounds are then the primary substrates that enter the chain of reactions known as GLYCOLYSIS.
13
149 reads
Glycolysis
When glucose reaches the cytoplasm of the cell, it undergoes a series of reactions to extract energy from its chemical bonds.
Glucose, a 6-carbon compound, reacts with ATP in this process, resulting in the formation of Glucose-6-phosphate.
12
162 reads
First Step - Glucose Phosphorylation
The first step as soon as the glucose enters the cell is that it undergoes immediate phosphorylation. So that it cannot diffuse out of the cell. This is because, on phosphorylation, the glucose becomes polar and hence cannot flow across the plasma membrane passively.
This step functions at the expense of an ATP. Glucose reacts with an inorganic phosphate available from ATP and converts into glucose 6-phosphate. i.e., a phosphate group is added to the 6th carbon of Glucose.
This reaction is catalyzed by the enzyme glucokinase or hexokinase.
# I wrote the reaction above in the picture.
9
97 reads
The only difference between GLUCOKINASE and HEXOKINASE is their kinetic property and efficiency. Glucokinase has the ability to convert more glucose into glucose 6-phosphate than comparison to hexokinase.
GLUCOKINASE V/S HEXOKINASE
9
99 reads
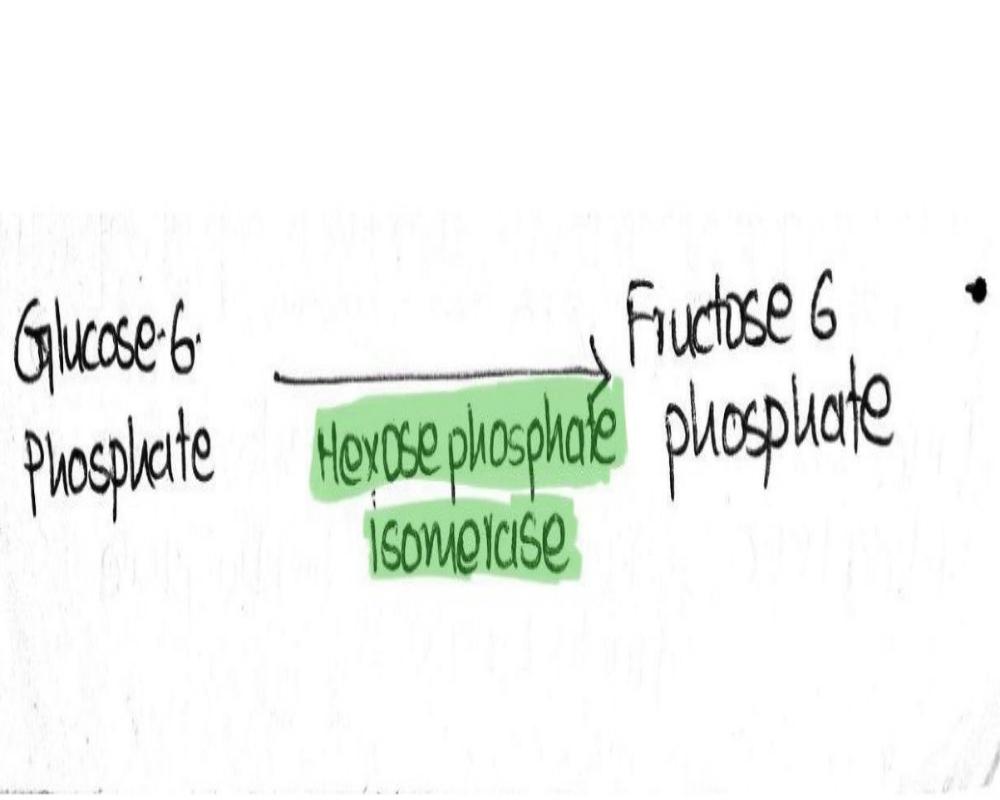
Second Step - Isomerization Of Glucose-6-phosphate (G-6-P)
Once glucose is converted into glucose 6-phosphate, it is then isomerized into fructose 6-phosphate. The basic reason behind this conversion is that the addition of one more phosphate into glucose is not possible, due to its cyclic structure. Where only one carbon atom is present outside of the ring unlike fructose where two carbon atoms are present on the outside of the ring. And hence, two phosphates can attach to a fructose ring.
Therefore, glucose-6-phosphate is first isomerized to fructose-6-phosphate for further phosphorylation under the category of hexose-phosphate isomerase.
9
59 reads
Third Step- Phosphorylation Of Fructose 6 Phosphate
Fructose 6-phosphate on reaction with another ATP results in the formation of fructose 1,6-bisphosphate.
This reaction is under the control of phosphofructokinase, which is also the pacemaker of this reaction.
At a time, this reaction proceeds in any one direction only. Therefore, called irreversible reaction.
9
55 reads
Fourth Step- Breaking of Fructose-1, 6-bisphosphate.
Fructose 1,6-bisphosphate, being highly active, breaks down into two three-carbon compounds, namely dihydroxyacetone phosphate (DHAP) and phosphoglyceraldehyde (G3P) under the activity of enzyme aldolase.
9
52 reads
5. DiHydroxyAcetonePhosphate Isomerization to PGAL.
Dihydroxyacetone phosphate, which was formed along with the formation of phosphoglyceraldehyde during the breaking of fructose 1,6-bisphosphate. Both of these triose sugars are capable of isomerizing into either forms, and hence DHAP gets isomerized into G3P. This reaction activity is under the control of enzyme triose-phosphate-isomerase. And then the DHAP is converted into G3P, which can now contribute in the formation of pyruvic acid.
9
49 reads
6. Partial Oxidation of PGAL—Phosphoglyceraldehyde.
The phosphoglyceride hence obtained undergoes partial oxidation. It reacts with an inorganic phosphate and results in the formation of 1,3-bisphosphoglyceric acid along with the synthesis of an NADH+H+.
Enzyme-
9
51 reads
7. Substrate Level Phosphorylation In 1, 3BPG
The 1,3-BPG hence obtained from the partial oxidation of G3P undergoes substrate-level phosphorylation. An inorganic phosphate is released by the substrate named 1,3-BPG which is taken by an ADP molecule and hence results in the formation of an ATP molecule.
This 1,3-BPG gets now converted into 3-phosphoglyceric acid along with the formation of an ATP (hence payoff phase).
Enzyme - 3PGKinase
9
48 reads
8. Conversion Of 3-PGA TO 2-PGA
The 3-phosphoglyceric acid hence obtained is now mutated to form 2-phosphoglyceric acid by the activity of enzyme mutase.
The position of phosphate is changed from 3rd carbon to 2nd carbon for the formation of Phosphoenolpyruvate.
9
53 reads
9. Dehydration Of 2-PGA to Form PEPA
The 2-phosphoglyceric acid hence obtained is now dehydrated to form phosphoenolpyruvate acid. This activity is under the control of enzyme enolase and result in the removal of a water molecule from the substrate.
9
50 reads
10. Formation of PYRUVATE
The phosphoenolpyruvate obtained is now under the activity of enzyme pyruvatekinase and is converted into pyruvic acid along with the formation of an ATP as a premium-product.
- The highly energetic phosphate bond of PEPA is transferred to the ADP molecule and results in ATP formation.
- Again, the substrate is providing the inorganic phosphate, and therefore this step involves Substrate level phosphorylation.
Enzyme - Pyruvate-Kinase
9
61 reads
IDEAS CURATED BY
CURATOR'S NOTE
Important for studies.
“
Similar ideas
Read & Learn
20x Faster
without
deepstash
with
deepstash
with
deepstash
Personalized microlearning
—
100+ Learning Journeys
—
Access to 200,000+ ideas
—
Access to the mobile app
—
Unlimited idea saving
—
—
Unlimited history
—
—
Unlimited listening to ideas
—
—
Downloading & offline access
—
—
Supercharge your mind with one idea per day
Enter your email and spend 1 minute every day to learn something new.
I agree to receive email updates